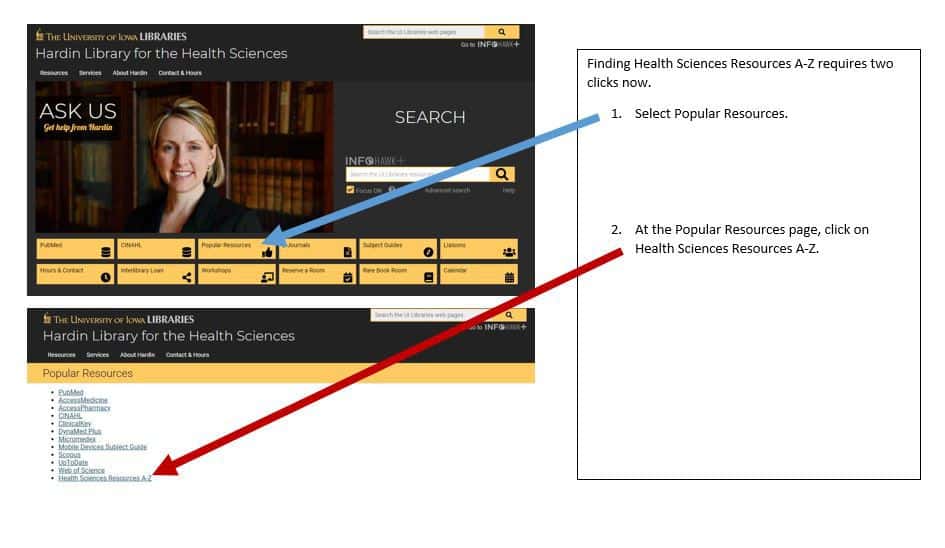
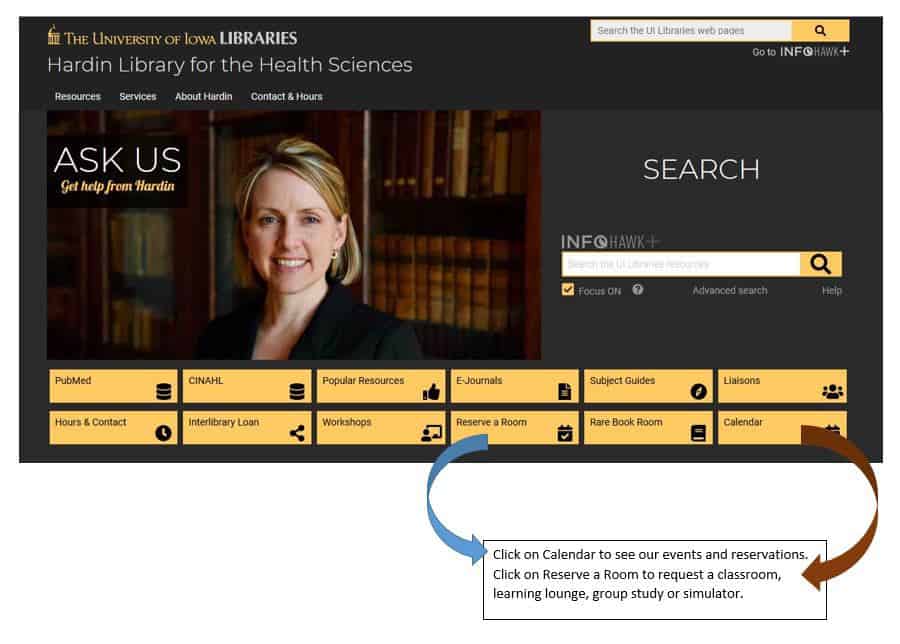
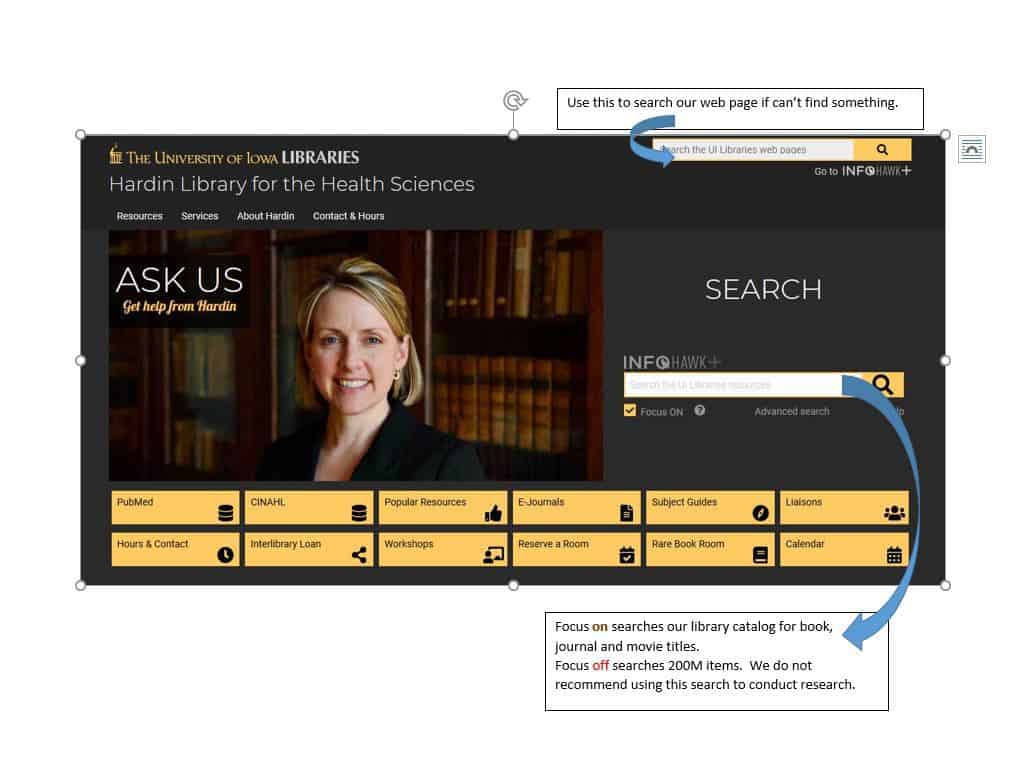
For those already using Endnote, this class teaches you to maximize the tool. From exporting and importing to syncing and sharing, this class will help you manage your own information seamlessly from desktop to mobile device and on the web. You’ll also learn about the Endnote options for sharing, so you can collaborate effectively.
Our summer workshops:
Wednesday, June 27, 1-2pm, Information Commons East, 2nd Floor, Hardin Library 
Thursday, July 26, 1-2pm, Information Commons East, 2nd Floor, Hardin Library
Register online or by calling 319-335-9151.
EndNote Desktop is available FREE from the UI Libraries to all graduate students, faculty and staff. Download your own copy.
Getting here: Easiest way to get to Hardin Library is by taking Cambus/Pentacrest Route and get off at the stop called VA Loop.
Individuals with disabilities are encouraged to attend all University of Iowa-sponsored events. If you are a person with a disability who requires a reasonable accommodation in order to participate in this program please call Janna Lawrence at 319-335-9871.







 EndNote is a reference management tool that helps you to easily gather together your references in one place, organize them, and then insert them into papers and format them in a style of your choosing.
EndNote is a reference management tool that helps you to easily gather together your references in one place, organize them, and then insert them into papers and format them in a style of your choosing.